Abstract
Introduction
Mutations in nucleophosmin (NPM1) in the presence of a normal karyotype in acute myeloid leukemia (AML) typically portend a favorable outcome and are present in approximately 28-35% of patients; however, its prognostic importance and effect on chemotherapy sensitivity is unclear in elderly patients (age ≥ 70). To this end, we investigated the impact of NPM1 mutation (NPM1+) on clinical outcomes in a large cohort of AML patients aged ≥ 70 years.
Methods
We analyzed an institutional database of 983 AML patients aged ≥ 70 years treated between 1995 and 2016. Response rates and overall survival (OS) were compared between NPM1+ and wildtype NPM1 (NPM1-) patients, with a focus on presence or absence of FLT3-ITD mutation (FLT3+ or FLT3-). The Kaplan Meier method was used for survival analysis and the log-rank test was used to determine significance (p-value ≤0.05). The World Health Organization (WHO) criteria were used to define complete response (CR) and complete response with incomplete hematologic recovery (CRi). Categorical data were analyzed by Fisher exact test and all calculated p-values are two-tailed.
Results
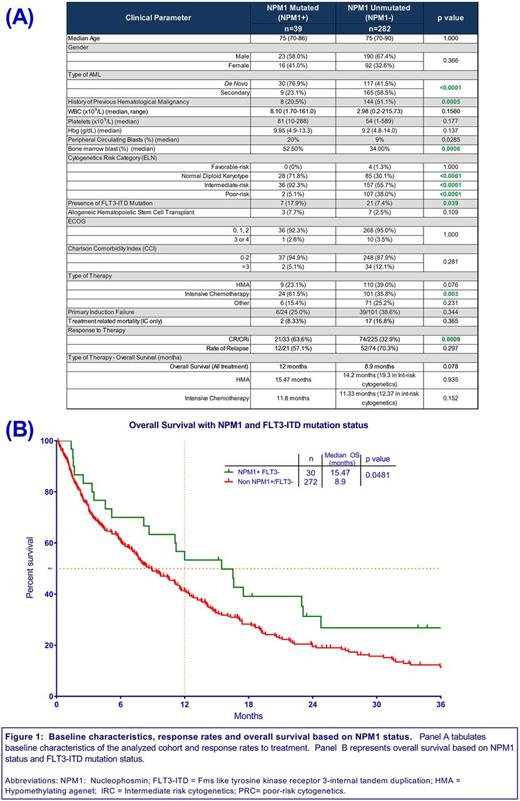
Of the 983 patients, 321 patients had NPM1 tested at diagnosis and a mutation was detected in 39 (12.1%) patients. Baseline characteristics of both NPM1+ and NPM1- cohorts are described in Figure 1A. Frequency of de novo AML was higher in NPM1+ vs NPM1- cohorts (76.9% vs. 41.5%, p<0.0001) and rate of antecedent hematological malignancy (AHM) was higher in the NPM1- cohort (51.1% vs. 20.5%, p=0.0005). Frequency of European LeukemiaNet (ELN) defined poor risk cytogenetics (PRC) was greater in NPM1- cohort compared to NPM1+ (38.0% vs. 5.1%, p<0.0001). Presence of FLT3+ was significantly higher in NPM1+ cohort (17.9% vs. 7.4%, p=0.039). More patients were treated with intensive chemotherapy (IC) in the NPM1+ cohort vs the NPM1- cohort (61.5% vs 35.8%, p=0.003), whereas the rate of hypomethylating agent (HMA) use was similar between the 2 cohorts.
A trend toward improved median overall survival (OS) was observed in the NPM1+ compared to the NPM1- cohort (12 mo vs 8.9 mo, p=0.078). However, patients with NPM1+/FLT3- genotype had superior OS compared to all other genotypes (15.5 mo vs 8.9 mo, p=0.0481 (Figure 1B). A higher rate of CR/CRi was noted in the NPM1+ group vs the NPM1- group (63.6% vs 32.9%, p=0.001). Amongst patients with intermediate-risk cytogenetics, the rate of CR/CRi was 62.5% in the NPM1+ group vs 38.7% in the NPM1- group (p=0.018).
Conclusion
Our data suggest that NPM1 mutation in older adults (aged ≥70 years) with AML, while less commonly encountered than in younger patients, retains prognostic importance and impacts response to treatment. The NPM1+ group had a lower incidence of baseline adverse prognostic factors (e.g. PRC, AHM), a high rate of response to therapy, and a trend toward superior survival, particularly in the NPM1+/FLT3- cohort. Although these findings should be validated in a larger cohort, our data suggest that the NPM1+ elderly AML patients represent a unique subgroup that warrants further study to optimize outcomes.
Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau. Sweet: Novartis Pharmaceuticals: Consultancy, Speakers Bureau; Ariad: Consultancy, Speakers Bureau; Pfizer: Consultancy; Karyopharm: Consultancy, Research Funding; Otsuka: Consultancy; Incyte: Research Funding. Lancet: BioSight: Consultancy; Erytech: Consultancy; Bio-Path Holdings: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Pfizer: Other: Institutional research funding, Research Funding; Celgene: Consultancy; Jazz Pharmaceuticals: Consultancy; Boehringer Ingelheim: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal